Gastroparesis is a syndrome characterized by delayed gastric emptying in the absence of obstruction leading to epigastric discomfort, abdominal distention, nausea, and vomiting, although it can also be asymptomatic.1 One of the causes of acute gastroparesis, which is not widely reported and is unfamiliar to many cardiologists, is gastroparesis secondary to pulmonary vein (PV) cryoablation, a technique that is available at over 50% of Spanish electrophysiology laboratories.2 Based on a case of gastroparesis, we have reviewed this syndrome.
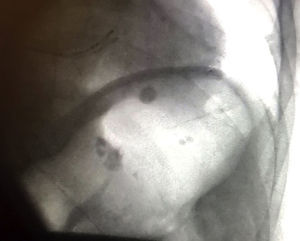
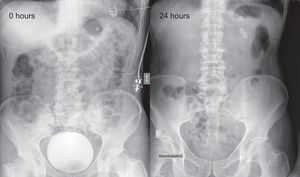
We report the case of a 59-year-old man, with a history of symptomatic paroxysmal atrial fibrillation despite the use of antiarrhythmic drugs, in whom we decided to perform PV cryoablation. During the procedure, performed under sedation with remifentanil and propofol, we observed a small left atrium on angiography (37 mm in the previous echocardiogram) with 4 independent PVs. We decided to use a 28-mm second-generation Medtronic Arctic Front cryoballoon. Due to poor occlusion and poor temperatures, 4 applications were required to isolate the left upper PV, 2 applications for the left lower PV, and another 2 for the right upper PV. In the right lower PV, which was the smallest in terms of the balloon, 3 applications were initially performed with a minimum temperature of –36°C; there was a transient loss of phrenic nerve capture during 1 application. The vein was finally isolated with a 23-mm balloon with a single application for 64 s at –60°C. The total cryoablation time was 26 minutes. The mean minimum temperature reached in all applications was –40 ± 16°C. No bonus applications were given after isolating the veins. On removal of the catheters, fluoroscopy revealed gastric dilation with air accumulation in the fundus (Figure 1). Although the patient only had abdominal distension with tympanism and flatulence, we decided to place him under observation in the coronary care unit for 6hours. Treatment was started with prokinetics (metoclopramide and erythromycin), antisecretory drugs, and a nil per os diet for the first 24hours. A nasogastric tube was not required. In the following 24hours, the symptoms and radiological changes were resolved (Figure 2). The patient remains asymptomatic.
Several studies have related acute gastroparesis following ablation to injury to the nerve fibers that innervate the pyloric sphincter and the stomach. These nerves mainly travel with the left vagal trunk through the anterior part of the esophagus, close to the posterior wall of the left atrium and the ostium of the PVs. Application of heat or cold in the posterior wall of the left atrium may damage these perioesophageal nerves, whether transiently or permanently, and may lead to gastroparesis.
Of the few published cases of gastroparesis following PV ablation, the majority occurred in patients undergoing radiofrequency ablation. Symptoms appeared within a few hours of the procedure, and spontaneously resolved with medical treatment and observation.3
In the case of PV cryoablation, a study by Guiot et al.4 in 66 patients showed, using endoscopic assessment of gastric motility as per protocol, that 9% of these patients had asymptomatic gastroparesis 24hours after the procedure. In a subsequent analysis, only the onset of phrenic nerve paralysis during the procedure was associated with a higher risk of gastroparesis, although this was transient. A more recent observational study prospectively compared the frequency of onset of gastroparesis among 104 patients undergoing PV cryoablation or radiofrequency.5 Six cases were detected in the cryoablation group (5% of the sample). Only one case was detected in the radiofrequency group. The patients who received cryoablation and developed gastroparesis had smaller atria (36 ± 2 mm) and lower mean temperatures were achieved (–51 ± 2.3°C). All these patients received medical treatment and none of them had residual symptoms at 6 months, with the exception of the patient with gastroparesis following radiofrequency.
We describe a complication of PV ablation that is not often associated with a history of PV cryoablation, particularly in cases of late onset and generally with good prognosis. Possible risk factors associated with cryoablation in our patient are low temperature, multiple applications, and the use of a large balloon in a small atrium. Ablation of the right lower PV was particularly complex, as it required 2 balloon sizes, and its anatomical relationship with the esophagus is unknown, as no imaging test was performed in advance, which could have been useful. Fluoroscopic observation of the stomach and distended intestinal loops is useful for the initial diagnosis. Confirmatory diagnosis could be obtained by means of gastric emptying scintigraphy in severe and/or uncertain cases, although this technique is generally unnecessary. Initial treatment is conservative with antisecretory drugs and prokinetics, and endoscopic treatment is reserved for more severe cases.